Lithium batteries are a type of rechargeable battery that uses lithium-ion technology. The most common types you will find in the market are the lithium-ion used in electric vehicles, lithium-iron phosphate used for off-grid and renewable energy applications, and lithium-metal polymer that you find in our portable devices such as phones. These batteries have a high energy density which means that they can store more power than other types of batteries with the same size or weight.
There are many different variants defined by their anode and cathode materials. But first let’s learn how they work.
How Lithium Batteries Work
Essentially, lithium batteries work by using an anode and a cathode made from different chemical combinations in order to generate electricity. The process of how the battery emits electrons is called oxidation or reduction.
The flow of the electric current depends on the ability of the oxygen atoms in the electrolyte to move around and react when they meet up with electrons from the outside solution or electrode. Lithium batteries often use a third substance called “electrolyte” which allows for this ion movement more effectively than water alone can.
In these types of cells, reducing agents such as metallic lithium provide electrons that create power while oxidizing agents such as copper oxide are used in order to extract those ions back which then starts all over again. This is often referred to as the “rocking chair mechanism”.
1. Lithium Cobalt Oxide (LiCoO2) — LCO
A Lithium Cobalt Oxide battery consists of a cobalt oxide cathode and a graphite carbon anode.
The advantage to these batteries is their high discharge rate capabilities, which makes them ideal for situations when large amounts of energy need to be discharged quickly, such as heavy machine tools or electric vehicles.
Another advantage is their stability at low temperatures since some other types of batteries are plagued by increased rates of self-discharge at below-freezing levels. Lithium cobalt oxide has also been shown to withstand more than twice the number of charge cycles
The drawback of LCO batteries is a relatively short life span, low thermal stability and limited load capabilities.
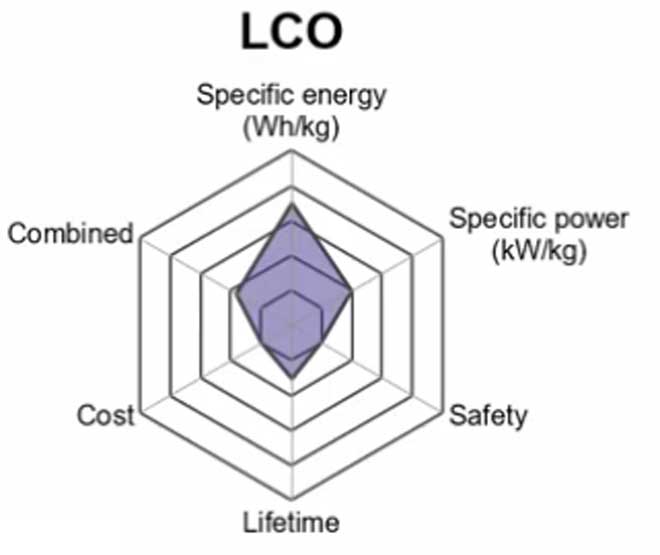
Li-cobalt should not be charged and discharged at a current higher than its C-rating.
It is still used in portable devices but the high price of cobalt means that it not practical for bigger applications such as electric vehicles.
2. Lithium Manganese Oxide (LiMn2O4) — LMO
The lithium manganese oxide battery or Li-MnO2 battery uses a lithium manganese oxide as cathode material. The battery also uses an electrolyte consisting of a mixture of ethers and phosphoric acid esters dissolved in the same solvent.
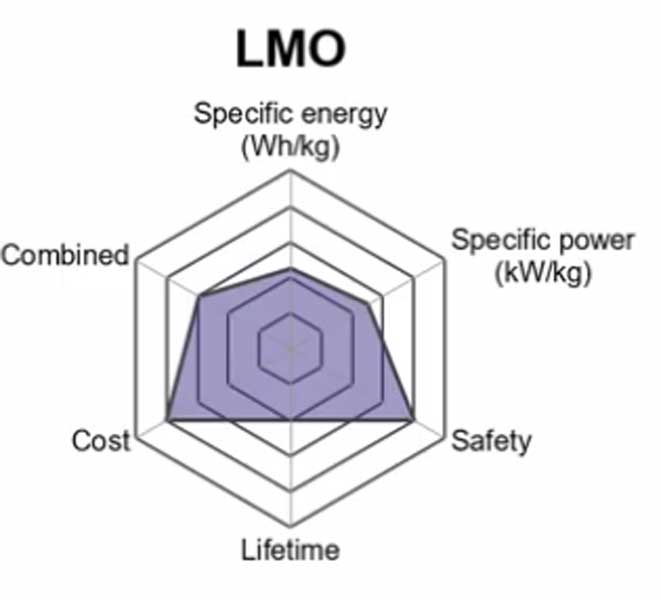
The advantages are low cost and good non-aqueous run time performance at high discharge rates (i.e., up to 10000 mAh/g). Disadvantages include environmental sensitivity and flammability. It has relatively flat voltage profile during discharge characteristic which limits its ability to control output current
3. Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) — NMC
Lithium Nickel Manganese Cobalt Oxide batteries (often just called LMO or LiNiCoMnO2) are a type of rechargeable lithium-ion battery. The cathode in this type of battery is a combination of nickel-manganese-cobalt (NMC).
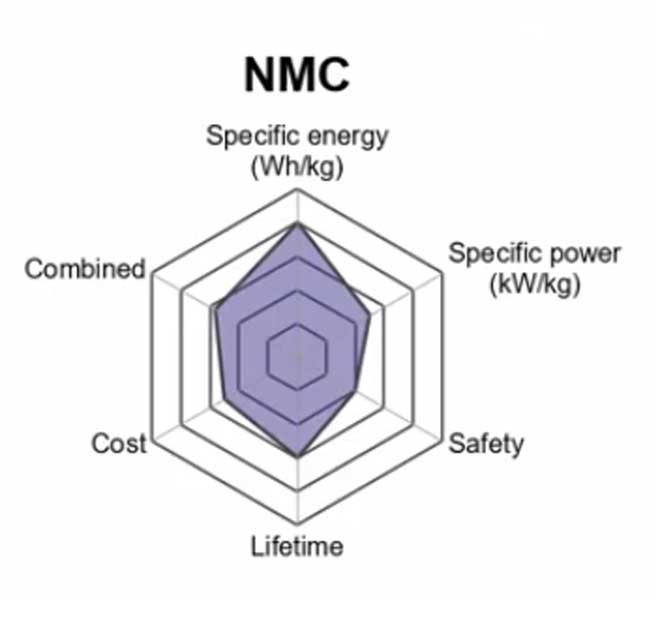
A lot of the benefits in this case are due to reduced production cost as well as reduced risk during operation but it also has to do with materials properties. MnO2 achieves such good performance because its low operating voltage makes it more tolerant to overcharge/over-discharge cycles while not impacting its specific energy levels as much when compared with other lithium batteries.
4. Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) — NCA
The Lithium Nickel Cobalt Aluminum Oxide battery or NCA is a further development of lithium nickel oxide; adding aluminum to the cathode gives the chemistry greater stability. They are often compatible with existing power storage hardware and charging stations.
Some companies Panasonic and their partners Tesla have been successful in addition Silicon to the Graphite anode that considerably increases the battery’s capacity. This has the drawback of the anode growing and shrinking.
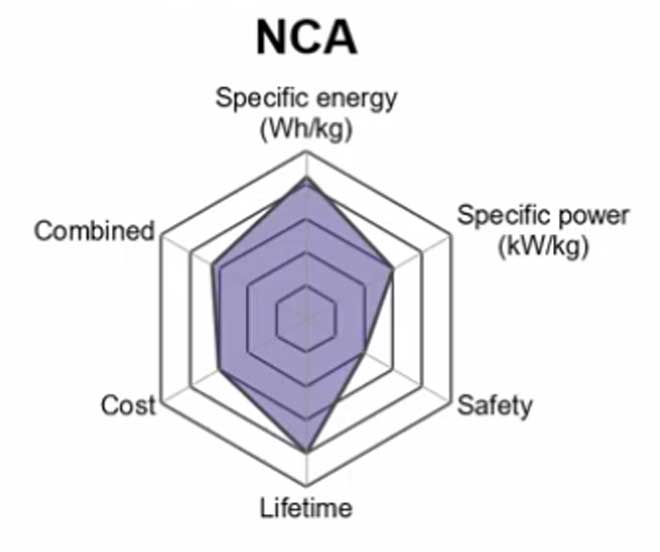
It uses a safe, nonflammable chemistry that offers significant weight savings over LiCoO2-based designs while addressing safety concerns related to previous options like LiMn2O4 or LiMnO2. This provides an additional computational advantage considering many applications will make use of the higher voltage (and corresponding efficiency) offered by the cathode material.
5. Lithium Iron Phosphate (LiFEP04) – LFP
A lithium Iron phosphate battery is a type of rechargeable battery that belongs to the group of lithium-ion chemistry. These batteries are common because they have a lower self-discharge rate and use of phosphate cathode material that provides increased safety due to their resistance to extreme temperatures.
The primary use for these batteries is in electric bikes, more specifically an e-bike or low-speed bike where long-range capability is not needed much. This makes this kind of battery ideal for daily commuting distances within the city limits.
Another advantage that it has over traditional lead acid batteries in cars is that it does not require special handling and disposal protocols which makes it the best choice for boats, golf carts, and solar power.
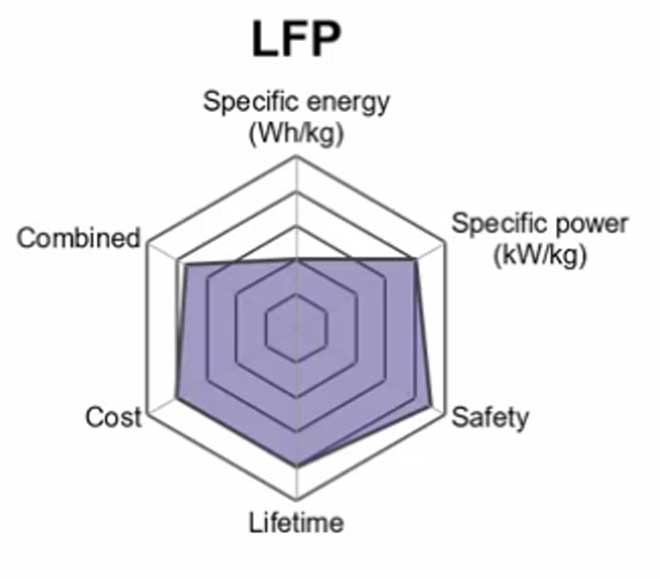
Lithium-iron phosphate has some advantages over lithium-ion because it’s safer to use in low temperatures and can be used as an emergency backup when there is no electricity available because it doesn’t require charging. However, this type of battery costs five times more than lithium-ion so it is not commonly used by consumers.
6. Lithium Titanate (Li2TiO3) — LTO
Lithium Titanate batteries are made of two different materials, one being lithium titanate as the anode and lithium manganese oxide or NMC as the Cathode.
Lithium Titanium oxide is more efficient at storing energy in an electrochemical cell. However, lithium titanium oxide has very poor high-temperature performance and would not be a viable contender to replace existing lead acid battery technology found in cars.
Lithium Titanate comes as white or greenish-blue pellets used for a variety of applications such as air-conditioning equipment, power tools and computers. The material can also be shaved into a powder to coat metals like copper which makes the resulting product conductive even if it’s encased in plastic for example. It can also be heated and pressed into shapes to make capacitors–which store electric charge—
Form Factors
Prismatic Cell
Prisms are a different kind of battery design that tries to make a battery last longer by having both charged and discharged circuits for each fully charged cycle. This is supposed to increase life twice as much as regular batteries. It’s about tackling the two major problems with traditional lithium-ion batteries, life span, and safety.
Lithium ions don’t like to be heated up too much or they’ll leak out violently and all hell breaks loose, so that’s where silicon comes in. Silicon is used in some areas of a prismatic battery because it expands more when heated than lithium does.
Hence creating insulation between outside air and whatever flammable material might be next
Cylindrical Cell
18650 lithium cells are cylindrical batteries with high energy density, and they have been proven to outperform other technologies.
Battery characteristics for Li-ion battery cells depend on the size of the cell as well as the chemistry it was designed with. 18650 is a size often used in laptops and power tools. These sizes are standardized according to their height, length, and total weight capacity (i.e., millimeters by mm by grams).
Of course, these measurements will largely depend on both target use as well as engineering design parameters. A smaller cell (e.g., 1500 mAh) may be able to be manufactured more cheaply than a larger cell (e.g., 3200 mAh), which makes sense
Pouch Cell
Lithium pouch cells are a type of rechargeable lithium ion cell.
Lithium is the lightest (lowest atomic weight) metal there is and has both advantages and disadvantages. Its advantages include a high volumetric energy density, which means it can pack in large amounts of energy into very small volumes – as does its cousin hydrogen.
However, because lithium’s ions are relatively small and have low mobility, making for inefficient packing of charged particles on a single electrode, this also means that more material must be used to extract charge from each volume – which again leads to high volumetric (energy density) but low gravimetric (mass per unit volume or total capacity) efficiency during discharging cycles of operation
Lithium-ion technology is continually seeking improvement in order to fully match the requirements of portable applications, electric vehicles, and off-grid banks. Several other technologies are in the pipeline and soon may be added to this article. Safety concerns, power, and energy density are some of the areas being sort for improvements in lithium batteries.
You are here
Leave a Reply